Berikut ini adalah langkah-langkah pengajuan permohonan baru untuk persetujuan etika penelitian yang melibatkan subjek manusia:
1. Estimasi Durasi Keseluruhan Proses
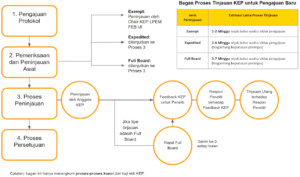
Mengacu pada prosedur operasional baku (SOP) KEP, bagian ini memberikan estimasi lama proses kaji etik dari batas waktu siklus pengajuan hingga keluarnya keputusan KEP.
Estimasi lama proses tinjauan exempt adalah:
- ± 1 – 2 Minggu sejak batas waktu siklus pengajuan
Estimasi lama proses tinjauan expedited adalah:
- ± 2 Minggu sejak batas waktu siklus pengajuan jika penelitian disetujui tanpa modifikasi/persyaratan tambahan
- ± 5 – 6 Minggu sejak batas waktu siklus pengajuan jika penelitian disetujui dengan modifikasi/persyaratan tambahan (dengan catatan respon dan revisi peneliti terhadap komentar KEP sudah dianggap cukup oleh peninjau)
Estimasi lama proses tinjauan full board adalah:
- ± 3 Minggu sejak batas waktu siklus pengajuan jika penelitian disetujui tanpa modifikasi/persyaratan tambahan atau penelitian ditolak
- ± 6 – 7 Minggu sejak batas waktu siklus pengajuan jika penelitian disetujui dengan modifikasi/persyaratan tambahan (dengan catatan respon dan revisi peneliti terhadap komentar KEP sudah dianggap cukup oleh peninjau).
2. Penyerahan Protokol Penelitian
Permohonan persetujuan etika kepada KEP dapat diajukan oleh seluruh Mahasiswa Pascasarjana, Dosen, dan Peneliti yang akan melaksanakan penelitian (baik yang berafiliasi maupun yang tidak berafiliasi dengan Universitas Indonesia).
Peneliti utama atau perwakilannya bertanggung jawab untuk menyerahkan dokumen dan formulir pengajuan yang telah diselesaikan beserta dengan lampiran yang diperlukan melalui email KEP LPEM FEB UI di kep@lpem-feui.org. Berikut adalah ketentuan subjek email pengajuan:
Format Subjek Email Pengajuan Baru
| Pengajuan protokol penelitian baru | Format:
Pengajuan Baru – (Nama Peneliti Utama) – (Nama Institusi) – (Bulan dan Tahun) Contoh: Pengajuan Baru – Gregory Mankiw – LPEM FEB UI – April 2021 |
KEP LPEM FEB UI menetapkan batas waktu pengajuan setiap bulannya. Setiap pengajuan, baik pengajuan baru, perpanjangan, maupun perubahan, yang masuk sampai dengan batas waktu pengajuan setiap bulannya akan segera diperiksa kelengkapannya oleh Administrator KEP. Pengajuan apapun yang diterima setelah batas waktu pengajuan setiap bulannya akan diproses pada bulan berikutnya.
3. Tenggat Waktu Pengajuan
Peneliti mempunyai kewajiban untuk memeriksa tanggal batas waktu pengajuan setiap bulannya di laman situs KEP. Peneliti dapat mengakses informasi mengenai tanggal-tanggal penting KEP ini di laman situs KEP dengan tautan berikut: Tanggal dan Tenggat Waktu Pengajuan.
KEP menyarankan peneliti untuk menyerahkan dokumen pengajuan sebelum tanggal batas waktu pengajuan sehingga seluruh dokumen perbaikan dapat ditinjau pada siklus pengajuan bulan yang sama. Jika informasi pada protokol penelitian dan dokumen pendukung tidak lengkap:
- Jika peneliti menyerahkan dokumen pengajuan sebelum tanggal batas waktu pengajuan, Administrator dapat meminta peneliti untuk melengkapi dokumen pengajuan dan peneliti mempunyai waktu sampai dengan batas waktu pengajuan di siklus yang sama untuk memperbaiki dokumen dan menyerahkan ulang dokumen yang sudah diperbaiki untuk diproses di siklus pengajuan bulan yang sama.
- Jika peneliti menyerahkan dokumen pengajuan tepat pada tanggal batas waktu pengajuan, Administrator tetap akan meminta peneliti untuk melengkapi dokumen pengajuan, akan tetapi dokumen yang sudah diperbaiki dan diserahkan ulang akan diproses pada siklus pengajuan bulan berikutnya.
Jika tidak ada kekurangan dokumen, KEP akan mengirimkan email konfirmasi penerimaan dokumen permohonan kepada peneliti. KEP menyarankan peneliti untuk melakukan follow-up ke KEP jika tidak menerima email konfirmasi penerimaan dokumen setelah 3 hari kerja semenjak tenggat waktu pengajuan.
Peneliti dapat memeriksa status pengajuan secara berkala pada laman situs KEP LPEM FEB UI dengan tautan berikut: Status Pengajuan.
4. Pemeriksaan dan Peninjauan Awal
Tahap ini terdiri dari dua.
- Tahap pertama adalah pemeriksaan oleh Administrator KEP. Jika permohonan tidak lengkap, maka permohonan akan dikembalikan kepada peneliti untuk dilengkapi. Jika permohonan lengkap, maka permohonan akan diteruskan kepada Chair KEP.
- Tahap berikutnya adalah prapeninjauan oleh Chair KEP untuk menentukan tim peninjau, tipe tinjauan (exempt, expedited, full board), dan prosedur tinjauan dengan potensi konflik kepentingan. Peneliti dapat mengetahui keputusan tipe tinjauan untuk pengajuannya di laman Status Pengajuan dalam waktu ± 1 minggu semenjak batas waktu siklus pengajuan.
5. Proses Peninjauan
a. Peninjauan KEP Terbatas untuk Kategori Pembebasan Tertentu (Exempt Review)
Exempt Review hanya dapat berlaku untuk beberapa kategori tertentu. Penentuan status pembebasan diputuskan oleh Chair dan dapat langsung disetujui jika memenuhi salah satu dari kategori pembebasan tertentu. Protokol penelitian dengan kategori Exempt Review tidak ditinjau oleh tim peninjau KEP.
Informasi lebih lanjut mengenai Peninjauan KEP Terbatas untuk Kategori Pembebasan Tertentu (Exempt Review) dapat di akses di laman situs KEP: Kategori Exempt dan Expedited Review.
b. Peninjauan Dipercepat (Expedited Review)
Peninjauan dipercepat adalah tipe peninjauan bagi penelitian subjek manusia yang melibatkan risiko tidak lebih dari minimal untuk subjek yang berpartisipasi.
Risiko minimal adalah kemungkinan atau besarnya bahaya atau ketidaknyamanan yang diantisipasi dalam penelitian yang diajukan tidak lebih besar dari yang biasa dihadapi dalam kehidupan sehari-hari atau selama pelaksanaan pemeriksaan tes fisik atau psikologis rutin.
Informasi lebih lanjut mengenai Peninjauan Dipercepat (Expedited Review) dapat di akses di laman situs KEP: Kategori Exempt dan Expedited Review.
c. Peninjauan Full Board
Peninjauan Full Board diberlakukan pada protokol penelitian dengan risiko lebih dari minimal. Setelah ditinjau oleh Anggota, protokol penelitian akan dipresentasikan kepada Dewan KEP pada saat rapat Full Board . Keputusan persetujuan atau penolakan protokol penelitian ditentukan pada saat rapat Full Board.
6. Keputusan Tinjauan
Keputusan hasil peninjuan dapat berupa salah satu dari pilihan keputusan berikut:
- Disetujui
Tim peninjau menilai penelitian dapat berjalan sesuai rencana peneliti tanpa modifikasi atau persyaratan tambahan.
- Disetujui? ?dengan? ?Modifikasi/Persyaratan? ?Tambahan?
Tim peninjau menilai rencana penelitian memerlukan beberapa modifikasi atau persyaratan tambahan sebelum dapat dijalankan. Peneliti wajib memenuhi modifikasi atau persyaratan tambahan tersebut atau berkomitmen untuk memenuhinya di masa mendatang (tergantung permintaan tim peninjau).
- Tidak Disetujui
Tim peninjau menilai rencana penelitian tidak layak etik. Tim peninjau wajib memberikan saran perbaikan agar rencana penelitian lebih layak etik. Peneliti dapat mengajukan permohonan kembali setelah rancangan penelitian diperbaiki. Keputusan ini hanya dapat diambil pada Rapat Full Board.
- Penangguhan
Dewan Anggota KEP memutuskan bahwa informasi dari peneliti kurang lengkap sehingga keputusan pengajuan ditangguhkan sampai dengan peneliti dapat memberikan informasi cukup yang memungkinkan KEP membuat keputusan. Keputusan ini hanya dapat diambil pada Rapat Full Board.
7. Tanggapan KEP terhadap Pengajuan
Jika tim peninjau memberikan keputusan setuju dengan catatan, Administrator KEP akan menyampaikan tanggapan terhadap pengajuan kepada peneliti dan peneliti berkewajiban untuk memberikan respon atas tanggapan ini. Mengacu pada prosedur operasional baku (SOP) KEP, berikut adalah estimasi waktu peneliti akan menerima tanggapan dari KEP:
- Expedited : ± 2 Minggu semenjak batas waktu siklus pengajuan
- Full Board : ± 3 Minggu semenjak batas waktu siklus pengajuan
Peneliti diberikan waktu 7 hari kerja atau maksimal 10 hari kalender untuk merespon dan mengirim revisi dokumen kepada KEP.
8. Peninjauan Respon dan Revisi
Proses peninjauan respon peneliti dan revisi dokumen adalah 3 (tiga) hari kerja untuk semua tipe tinjauan.
PENTING: Jika respon peneliti datang lebih cepat, KEP akan mengupayakan agar periode peninjauan ulang juga akan dimajukan.
9. Proses Persetujuan
Jika respon peneliti sudah dianggap cukup oleh tim peninjau KEP, maka pengajuan dapat berlanjut ke proses persetujuan. Surat Keputusan KEP akan dikirimkan kepada peneliti dalam waktu ± 1,5 minggu semenjak berakhirnya periode respon peneliti terhadap tanggapan KEP.
Surat Keputusan KEP berlaku selama kurun waktu yang disesuaikan dengan tingkat risiko, namun periode maksimum sampai dengan 1 tahun (365 hari) sejak dikeluarkannya keputusan. Peneliti juga memiliki tanggung jawab untuk mengingat tanggal berakhirnya persetujuan KEP.
10. Peninjauan Lanjutan dan Perpanjangan Persetujuan
KEP LPEM FEB UI akan melakukan peninjauan ulang terhadap penelitian yang melalui peninjauan dipercepat atau peninjauan dewan penuh setidaknya selama satu kali setiap tahun (dalam 365 hari sejak pemberian persetujuan sebelumnya). KEP memiliki wewenang untuk melakukan peninjauan yang lebih sering dari itu, tergantung dari penilaian KEP terhadap rasio risiko dan manfaat penelitian.
Informasi lebih lanjut mengenai proses pengajuan pembaharuan persetujuan dapat di akses di laman situs KEP: Perpanjangan Persetujuan.


 English
English