I. Penyerahan Laporan Kejadian
Peneliti utama atau perwakilannya bertanggung jawab untuk mengajukan laporan kejadian ke KEP. Waktu pelaporan yang KEP rekomendasikan bervariasi tergantung pada sifat dan masalah yang dilaporkan. Oleh karena itu, KEP menggunakan acuan berikut.
- Masalah yang tidak diantisipasi yang merupakan kejadian merugikan (unanticipated adverse event) harus dilaporkan ke KEP dalam kurun waktu paling lambat 48 jam setelah peneliti mengetahui kejadian tersebut;
- Masalah yang tidak diantisipasi lainnya yang bukan termasuk kejadian merugikan harus dilaporkan ke KEP dalam kurun waktu 10 hari kerja setelah peneliti mengetahui kejadian tersebut.
Peneliti dapat mengajukan laporan kapan saja, yang berarti proses peninjauannya pun dapat terjadi di luar siklus reguler.
Peneliti yang ingin mengajukan perubahan protokol perlu menyerahkan dokumen berikut:
Dokumen dikirim ke alamat email kep@lpem-feui.org dengan subjek: Laporan Kejadian Yang Tidak Diantisipasi – (nomor protokol)
KEP merekomendasikan peneliti memasukkan informasi berikut saat melaporkan kejadian merugikan sebagai masalah yang tidak diantisipasi kepada KEP:
- informasi yang sesuai untuk protokol penelitian, seperti judul, nama peneliti, dan nomor proyek KEP;
- deskripsi rinci tentang peristiwa, insiden, atau hasil yang merugikan;
- penjelasan tentang dasar untuk menentukan bahwa kejadian, insiden, atau hasil yang merugikan merupakan masalah yang tidak diantisipasi;
- deskripsi tentang setiap perubahan pada protokol atau tindakan korektif lainnya yang telah diambil atau diusulkan sebagai tanggapan atas masalah yang tidak diantisipasi.
II. Penjelasan Umum
A. Masalah yang Tidak Diantisipasi (Unanticipated Problem)
Masalah yang tidak diantisipasi adalah permasalahan yang secara tidak terduga melibatkan risiko terhadap subjek penelitian atau orang lain. Suatu kejadian dapat digolongkan sebagai masalah tidak diantisipasi jika:
- tidak terduga berdasarkan prosedur penelitian yang telah disetujui KEP dan karakteristik populasi subjek yang diteliti;
- terkait atau mungkin terkait dengan partisipasi subjek dalam penelitian; dan
- menempatkan subjek atau orang lain pada risiko bahaya yang lebih besar (termasuk bahaya fisik, psikologis, ekonomi, atau sosial) daripada yang diketahui sebelumnya.
Penting untuk digarisbawahi di sini adalah konsep risiko. Risiko adalah kemungkinan atau potensi bahaya atau kerugian, tetapi bukan berarti kejadian bahaya atau kerugian itu sendiri.
B. Kejadian Merugikan (Adverse Event)
Kejadian merugikan adalah setiap kejadian yang mencakup kerugian fisik dan psikologis pada subjek manusia, termasuk tanda-tanda abnormal, gejala, atau penyakit. Kejadian merugikan bisa jadi terkait maupun tidak terkait dengan partisipasi subjek dalam penelitian.
C. Hubungan antara Masalah yang Tidak Diantisipasi dan Kejadian Merugikan
Hubungan antara kedua hal ini dapat dijelaskan dengan Diagram Venn di bawah.
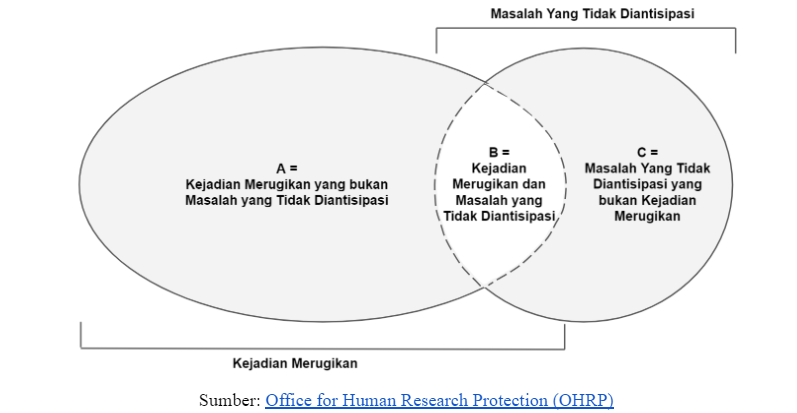
KEP mewajibkan peneliti untuk melaporkan kejadian pada area B (kejadian merugikan yang tidak diantisipasi) dan area C (masalah yang tidak diantisipasi yang bukan merupakan kejadian merugikan) yang terjadi selama penelitian berlangsung.
D. Kejadian Merugikan yang Tidak Diantisipasi (Unanticipated Adverse Event)
Kejadian merugikan yang tidak diantisipasi adalah kejadian yang memenuhi definisi kejadian merugikan dan masalah yang tidak diantisipasi.
-
Menentukan apakah suatu kejadian merugikan tidak diantisipasi
Kejadian merugikan dapat dianggap tidak diantisipasi jika terjadi pada paling tidak satu orang subjek yang berpartisipasi dalam penelitian dan sifat, keparahan, atau frekuensinya tidak konsisten dengan salah satu dari:
- Risiko kejadian merugikan yang diketahui atau dapat diperkirakan terkait dengan prosedur penelitian yang dijelaskan dalam (a) dokumen terkait protokol, seperti protokol penelitian yang disetujui KEP, brosur peneliti ??yang berlaku, dan dokumen Persetujuan Setelah Penjelasan (PSP) yang disetujui KEP, dan (b) sumber informasi lain yang relevan, seperti pelabelan produk dan sisipan kemasan; atau
- Perkembangan alami dari penyakit, kelainan, atau kondisi yang mendasari subjek dan profil faktor risiko predisposisi subjek.
Contoh kejadian merugikan yang tidak diantisipasi menurut definisi ini adalah sebagai berikut:
-
- Seorang peneliti hendak melakukan penelitian terhadap mahasiswa mengenai pengalaman sekolah usia dini dengan menggunakan metode survei. Penelitian ini telah melalui kaji etik dan dinilai tidak melibatkan risiko lebih dari minimal terhadap subjek. Namun, saat survei dilakukan, salah satu subjek menunjukkan suatu reaksi psikologis yang cukup intens yang ditandai dengan perubahan suasana hati dan perasaan yang tertekan tanpa adanya intervensi oleh peneliti. Setelah dilakukan evaluasi lebih lanjut, peneliti menganggap bahwa reaksi psikologis tersebut disebabkan oleh pertanyaan survei yang memicu ingatan tentang kekerasan fisik yang dialami oleh subjek. Perlu digarisbawahi, peneliti tidak menyangka bahwa reaksi seperti itu dipicu oleh pertanyaan survei.
-
Menentukan apakah kejadian merugikan terkait atau mungkin terkait dengan partisipasi dalam penelitian
Kejadian merugikan dapat disebabkan oleh satu atau lebih hal berikut:
- Prosedur dalam penelitian;
- Penyakit, kelainan, atau kondisi yang mendasari subjek; atau
- Keadaan lain yang tidak terkait dengan penelitian.
Secara umum, kejadian merugikan yang disebabkan oleh nomor (1) akan dianggap terkait dengan partisipasi dalam penelitian, sedangkan kejadian merugikan yang disebabkan semata-mata oleh nomor (2) atau nomor (3) akan dianggap tidak terkait dengan partisipasi dalam penelitian.
-
- Sebagai contoh, seorang siswa yang menerima perundungan dari siswa lain sebagai akibat dari partisipasinya dalam penelitian mengenai disleksia merupakan kejadian merugikan yang terkait dengan partisipasi dalam penelitian karena kejadian tersebut memang disebabkan oleh prosedur yang mengharuskan siswa dengan karakteristik khusus untuk terlibat dalam penelitian. Sedangkan jika subjek dengan kelainan bipolar mengalami gejala depresi saat penelitian mengenai persepsi terhadap produk kecantikan (topik yang tidak ada hubungannya sama sekali dengan kondisi yang mendasari subjek) berjalan bukan merupakan kejadian merugikan yang terkait dengan partisipasi dalam penelitian karena kejadian tersebut merupakan akibat yang ditimbulkan dari penyakit, kelainan, atau kondisi yang mendasari subjek.
-
Menentukan apakah kejadian merugikan menempatkan subjek atau orang lain pada risiko bahaya yang lebih besar daripada yang diketahui sebelumnya
Kejadian merugikan dianggap menempatkan subjek atau orang lain pada risiko bahaya yang lebih besar daripada yang diketahui sebelumnya jika suatu kejadian merugikan berpotensi menyebabkan risiko bahaya yang sifat, keparahan, atau frekuensinya lebih tinggi daripada yang diidentifikasi pada protokol penelitian.
Kejadian merugikan ini dibedakan menjadi 2 (dua) jenis kejadian:
Kejadian Darurat
KEP mendefinisikan kejadian merugikan darurat sebagai setiap kejadian yang:
- Berujung kematian subjek;
- Mengancam nyawa subjek (menempatkan subjek dalam risiko kematian yang sangat dekat ketika kejadian terjadi;
- Berujung pada subjek harus menjalani rawat inap di rumah sakit atau memperpanjang periode rawat inap yang sedang berjalan;
- Berujung pada disabilitas yang signifikan atau menerus pada subjek;
- Berujung pada kelainan bawaan (congenital anomaly)/kecacatan lahir;
- Berdasarkan penilaian medis yang tepat, kejadian bisa membahayakan kesehatan subjek dan mungkin membutuhkan intervensi medis atau operasi untuk mencegah satu atau beberapa konsekuensi kejadian yang sudah disebutkan sebelumnya.
Kejadian Tidak Darurat
Kejadian merugikan tidak darurat adalah kejadian merugikan yang tidak termasuk dalam Kejadian Darurat namun tetap berpotensi menyebabkan risiko bahaya fisik atau psikologis yang lebih tinggi daripada yang diketahui sebelumnya.
Berikut merupakan contoh dari kejadian tidak diantisipasi yang dapat dikategorikan sebagai kejadian tidak darurat, namun tidak terbatas pada:
- Perubahan kondisi subjek (kesehatan fisik, mental, dsb) yang memengaruhi kesanggupan subjek untuk berpartisipasi dan tidak dapat diselesaikan oleh peneliti.
- Pelanggaran terhadap kerahasiaan atau privasi subjek yang melibatkan potensi risiko bagi subjek atau orang lain, seperti laptop peneliti yang berisi data subjek yang dapat diidentifikasi telah dicuri.
- Adanya informasi baru yang menunjukkan kemungkinan perubahan pada penilaian risiko-manfaat atau penangguhan penelitian karena risiko yang baru diketahui.
- Penahanan subjek oleh pihak kepolisian saat penelitian berlangsung.


 English
English