Stages of applying for a continuing review approval:
The application for continuing review approval must be submitted two months (two cycles of KEP submission) before the expiration date.
- E.g.: KEP approval expires on December 3, 2022, then the researcher must apply for an extension in the submission cycle of October 2022.
Conducting research whose approval has expired may result in the termination of KEP’s approval of the research protocol.
- Continuing Review Approval Procedure
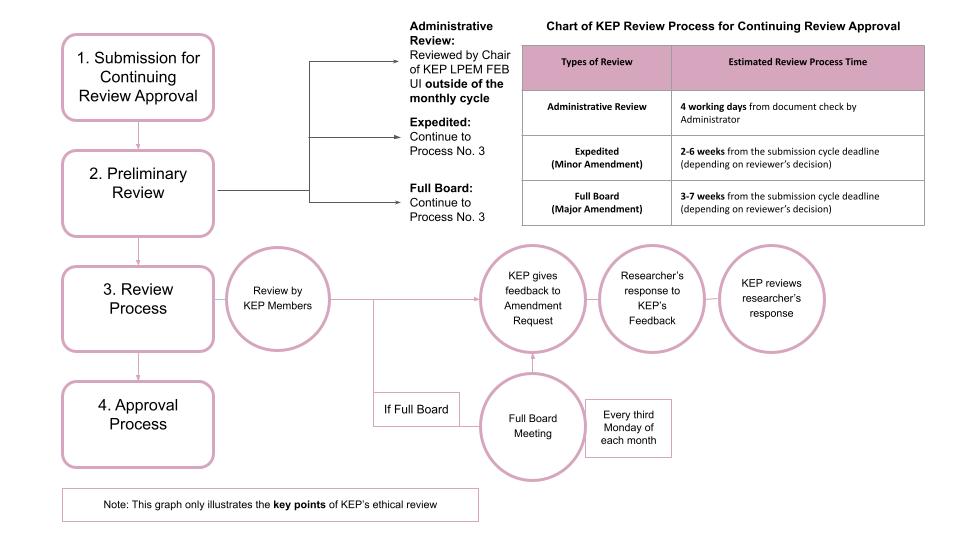
Continuing review approval is only required for expedited and full board research protocols that are still or will carry out subject recruitment activities, collecting and analyzing data, and storing personal identifiable information (PII / private identifiable information).
The review procedure for continuing review approval only requires an administrative review, as long as there are no changes, both minor and major changes, to the research plan as stated in the previously approved research protocol.
Researchers must complete the Continuing Review Approval Form accompanied by:
- Newly updated instruments and other instruments (if any) to be used in research
- Copies of informed consent forms that have been used for data collection
- Other supporting documents (if any)
Send the continuing review approval documents above to the e-mail address kep@lpem-febui.org with the subject: Application for Continuing Review Approval – (Registration Number)

