I. Submission of Incident Reporting
The Principal Investigator or their representative is responsible for submitting an incident report to KEP. The reporting time that KEP recommends varies depending on the nature of the issue being reported. KEP uses the following references.
- Unanticipated problems that are unanticipated adverse events should be reported to KEP no later than 48 hours after the researcher becomes aware of the event;
- Other unanticipated problems that are not adverse events should be reported to KEP within 10 business days after the researcher becomes aware of the event.
Researchers can submit reports at any time, which means the review process can occur outside of the regular cycle.
Researchers who wish to apply for a report will need to submit the following documents:
Documents should be sent to the email address kep@lpem-febui.org with the subject: Unanticipated Event Report – (protocol number)
KEP recommends researchers to include the following information when reporting adverse events as unanticipated issues to KEP:
- appropriate information for the research protocol, such as the title, researcher name, and KEP project number;
- a detailed description of the adverse event, incident, or outcome;
- an explanation of the basis for determining that the adverse event, incident, or outcome is an unanticipated problem;
- a description of any changes to the protocol or other corrective actions that have been taken or proposed in response to the unanticipated problem.
II. General Information
A. Unanticipated Problems
An unanticipated problem is a problem that unexpectedly involves a risk to the research subject or others. An event may be classified as an unanticipated problem if:
- it is unforeseen based on the KEP approved research procedures and the characteristics of the subject population under study;
- is or may be related to the subject’s participation in the research; and places the subject or others at greater risk of harm (including physical, psychological, economic, or social harm) than previously known.
Important to highlight here is the concept of risk. Risk is the possibility or potential of harm or loss, but not necessarily the occurrence of harm or loss itself.
B. Adverse Event
An adverse event is any event that includes physical and psychological harm to a human subject, including abnormal signs, symptoms, or illness. Adverse events may or may not be related to the subject’s participation in the study.
C. Relationship between Unanticipated Problems and Adverse Events
The relationship between these two can be explained with the Venn Diagram below.
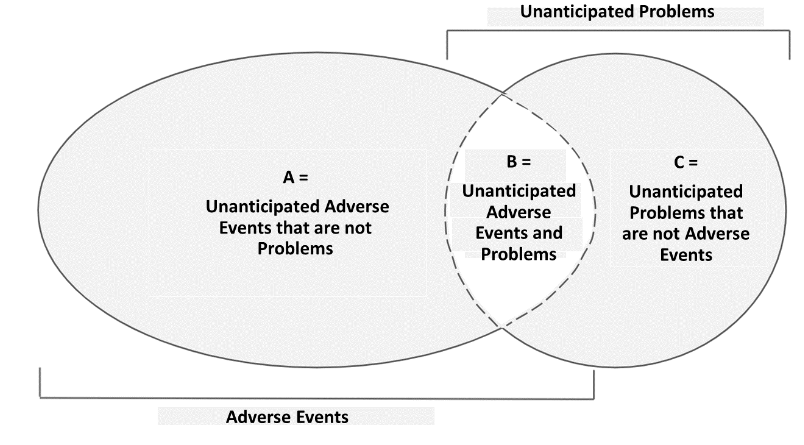
KEP requires researchers to report events in area B (unanticipated adverse events) and area C (unanticipated problems that are not adverse events) that occur during the study.
D. Unanticipated Adverse Event
An unanticipated adverse event is an event that meets the definition of an adverse event and an unanticipated problem.
III. Determining whether an adverse event is unanticipated
An adverse event may be considered unanticipated if it occurs in at least one subject participating
in the study, and its nature, severity, or frequency is not consistent with any of the following:
- The known or foreseeable risk of the adverse event associated with the research procedures described in (a) protocol-related documents, such as KEP-approved research protocols, existing researcher’s brochures, and KEP-approved informed consent documents, and (b) other relevant sources of information, such as product labeling and package inserts; or
- The natural progression of the subject’s underlying disease, disorder, or condition and the subject’s predisposing risk factor profile.
Example of unanticipated adverse events under this definition is as follows:
- A researcher wishes to conduct a study of university students regarding their early schooling experiences using survey methods. The study has undergone ethical review and is judged to involve no more than minimal risk to the subjects. However, during the survey, one of the subjects showed an intense psychological reaction characterized by mood swings and distress without any intervention by the researcher. Upon further evaluation, the researcher considered that the psychological reaction was caused by the survey questions triggering memories of physical violence experienced by the subject. It should be noted that the researcher did not expect that such a reaction was triggered by the survey questions.
IV. Determining whether an adverse event is or may be related to participation in the research
Adverse events may be caused by one or more of the following:
- Procedures in the study;
- The subject’s underlying disease, disorder, or condition; or
- Other circumstances unrelated to the study.
In general, adverse events caused by number (1) will be considered related to participation in the research, while adverse events caused solely by number (2) or number (3) will be considered unrelated to participation in the research.
For example, a student receiving bullying from other students as a result of participating in a research study on dyslexia is an adverse event related to participation in the study because the event is indeed caused by the procedures that require students with specific characteristics to be involved in the study. Whereas if a subject with a bipolar disorder experiences depressive symptoms while participating in a study on perceptions of beauty products (a topic that has nothing to do with the subject’s underlying condition) , it is not considered an adverse event related to participation in the study because the event is a result of the subject’s underlying illness, disorder or condition.
V. Determining whether an adverse event places the subject or others at greater risk of harm than previously known
An adverse event is considered to place a subject or other person at greater risk of harm than previously known if an adverse event has the potential to cause a risk of harm that is higher in nature, severity, or frequency than identified on the research protocol.
This type of adverse events can be divided into 2 (two) types of events:
Emergency Events
KEP defines an emergency adverse event as any event that:
- Leads to the death of the subject;
- Threatens the subject’s life (puts the subject at imminent risk of death when the event occurs);
- Leads to the subject’s hospitalization or extends an ongoing period of hospitalization;
- Leads to significant or persistent disability of the subject;
- Result in congenital anomaly/birth defect;
- Based on appropriate medical judgment, the event may jeopardize the health of the subject and may require medical or surgical intervention to prevent one or more of the aforementioned consequences of the event.
Non-Emergency Events
Non-emergency adverse events are adverse events that do not fall under Emergency Events but still have the potential to cause a higher risk of physical or psychological harm than previously known.
The following are examples of unanticipated events that may be categorized as non-emergency events, but are not limited to:
- Changes in the subject’s condition (physical health, mental health, etc.) that affect the subject’s ability to participate and cannot be resolved by the researcher.
- Violation of the subject’s confidentiality or privacy involving potential risk to the subject or others, such as the researcher’s laptop containing the subject’s identifiable data has been stolen.
- The existence of new information indicating a possible change to the risk-benefit assessment or suspension of the study due to newly recognized risks.
- Detention of the subject by the police while the study is in progress.

