The following are the steps for submitting amendment request for ethical approval of research involving human subjects:
Researchers are required to apply for amendment request first before carrying out changes in the research study. Implementing changes in the research without obtaining prior amendment request approval from KEP is an ethical violation and may result in termination of KEP’s ethical approval of the research protocol.
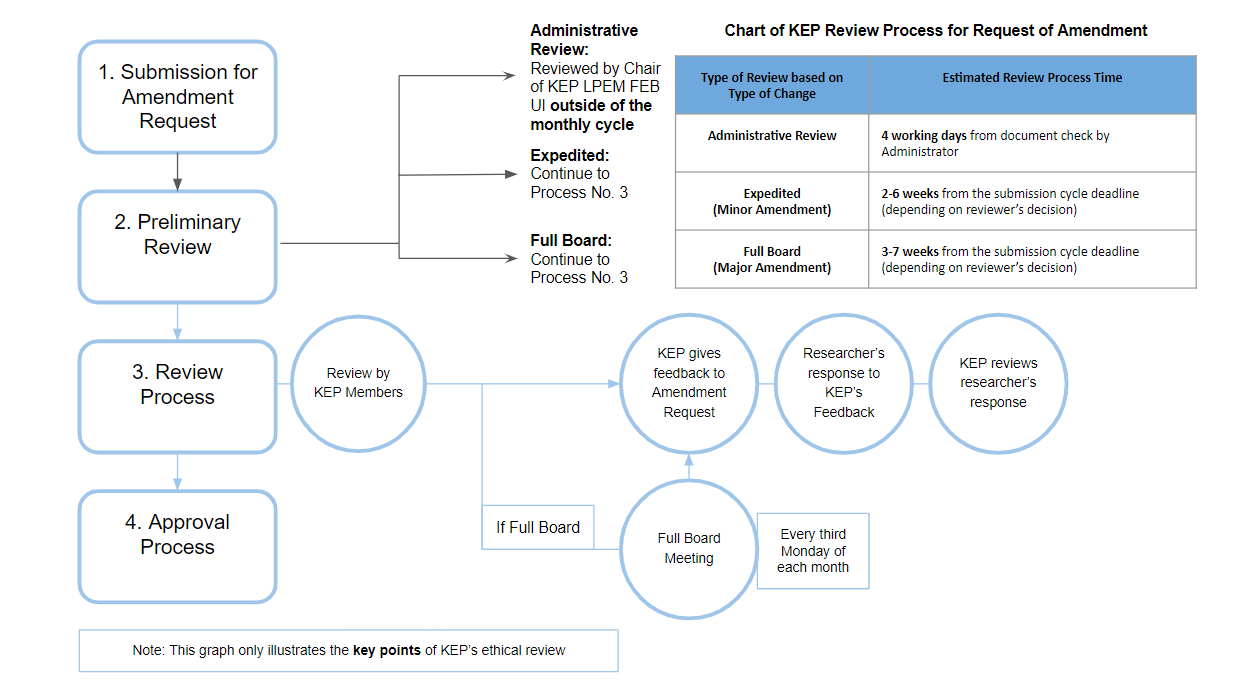
I. Summary of Amendment Procedure
In short, the procedure for submitting and reviewing an amendment is the same as the procedure for submitting and reviewing a new protocol. Minor amendments will get an expedited review and major amendments will get a full board review. Researchers are still required to submit an application before the deadline for submitting a cycle. Amendment applications that are submitted after the deadline will be processed in the next cycle.
KEP may grant exceptions to the cycle rule for administrative amendment. This type of amendment can be reviewed and approved outside of the regular monthly KEP cycle.
Researchers wishing to apply for amendment request will need to submit the following documents:
- Request for Amendment in Research Protocol Form
- The document(s) you want to change. Researchers are required to cross out the parts that would like to be deleted and highlight the part(s) that is proposed to be changed/added in yellow
Send the documents to KEP’s e-mail address kep@lpem-febui.org with the subject: Request for Amendment to Research Protocol – (Registration Number)
II. Types of Amendment
A. Administrative Amendment
- Definition, Categories, and Examples
Very minor changes that do not increase risk and affect the subjects and study design of the study in any way. The following are the categories of amendments that may be subject to administrative review:
i. Editorial changes that do not alter the meaning of a sentence/paragraph such as correcting spelling, typography, translation, grammar, and adding a few sentences with the intention of clarifying, on instruments, consent forms, recruitment materials, or other documents that are directly related to the research subjects. Examples:
-
- Adding no more than two sentences to the consent form
- Adding choice options to non-sensitive multiple choice questions
ii. Changes in procedures, whether in experiments, recruitment, data collection, or other processes throughout the course of the study, which in no way increase the risk of research subjects or change the research design significantly. Examples:
-
- Changing recruitment promotion media from brochures to posters without any significant content changes
- Adding banners as one of the media for recruitment promotions at locations previously approved by KEP
iii. Addition or change the research team members outside of the Principal Researcher and Associate Researcher. Example:
-
- Addition and/or change of research assistants, surveyors, transcript writers, data officers
iv. Changes in compensation for research subjects that are not significant enough to create coercion and/or undue influence on the subjects. Examples:
-
- Changes in the form of compensation with the same nominal value: to be in the form of goods from initially cash, or vice versa.
- Changing the form of compensation items that will be given to the subject from a pen to a pencil/marker
- Insignificantly increasing the value of compensation not significantly, from Rp. 25,000 per respondent to Rp. 30,000 per respondent
v. Additions and/or changes to the research team members as long as they do not have a negative effect on the competence and credentials of the research team. Examples:
-
- Adding a co-investigator
- The co-investigator leaves the team as long as it doesn’t negatively affect the team’s competence and credentials.
- Changes in roles, either vertically, such as co-investigators becoming principal investigators and principal investigators becoming co-investigators, or horizontally, such as exchanging roles between those in charge of collecting data and analyzing data.
vi. One small change from one of the categories of Minor Amendment.
Important Note:
-
- If the amendment request includes more than one category of Minor Amendment, then the research is not entitled to this category.
- This category does not apply to amendments that fall into the category of Minor Changes No. V.
vii. Minor changes from any of the categories may be subject to administrative review if the study protocol was initially approved through the exempt review procedure.
B. Minor Amendment
- Definitions, Categories, and Examples
The type of amendment that does not significantly affect the assessment of the risks and benefits of the study and does not substantially change the purpose or design of the study. Here are the categories of minor amendments:
i. Minor changes in consent form. Example:
-
- Adding more than two sentences and/or adding a new section to the consent form.
ii. Minor changes to recruitment procedures, recruitment materials or submission of new recruitment materials to be used according to the pre-approved recruitment methods. Examples:
-
- Adding banners as one of the recruitment promotion media in locations that have not previously been approved by KEP.
- Changed the recruitment procedure from canvassing to distributing online registration forms
iii. Minor changes to the research instrument. Example:
-
- Substantial changes that do not significantly increase the time and burden of subject participation, such as adding questions, changing the form of questions, changing the way of asking questions or extracting information from the subject, as long as these questions are non-sensitive.
iv. Increasing the number of samples as long as it does not negatively change the risk/benefit ratio of the study. Examples:
-
- Insignificantly adding samples from the same community group. For example, from 100 respondents to 120 respondents.
- Adding research subjects from community groups that have not previously been approved by KEP, as long as the group is not a vulnerable group
v. Minor changes to the study design. Examples:
-
- Changes in recruitment plans, data collection, interventions/experiments, and other procedures that are directly related to the research subjects of an ongoing research. If KEP assesses that these changes are still relatively minor, it may be reviewed through expedited review.
Note: If the amendment request falls into this category, the research is not entitled to administrative review category No. VI even though these changes are the only changes in the protocol.
C. Major Amendment
- Definition, Categories, and Examples:
The type of change that significantly affects the assessment of the risks and benefits of the study or substantially changes the purpose or design of the study. Here are the categories of major amendment:
i. Changes that significantly alter the risk/benefit ratio of the study or specifically increase the subject’s risks. Examples:
-
- Changes that affect the safety and physical or mental health of research subjects, such as adding sensitive questions, adding or reducing experimental procedures, interventions, or data collection (such as adding recording procedures)
- Substantial changes to the subject’s compensation that could be considered coercive or inappropriate when compared to the level of risk involved
ii. Changes to the inclusion and exclusion criteria that impact the risk/benefit ratio of the study
-
- Changing the inclusion or exclusion criteria that results in the research subjects being screened as vulnerable groups
iii. Significant changes in the study design. Examples:
-
- Addition of a new population that affects research risk, such as a vulnerable population
- Methodological changes, such as significant changes or additions to data collection methods
- Changes in experimental/intervention procedures that significantly affect the physical and mental safety of research subjects
- Changes in inclusion/exclusion criteria and/or subject recruitment procedures that have a major impact on the research risk/benefit ratio
iv. Significant changes to the consent form or other related documents that are directly related to the research subjects. Examples:
-
- Changing consent approval procedures from written to verbal or requesting an exempt for consent or an exempt for consent documentation
- Adding questions that significantly increase time and burden of research subjects during the interview, such as sensitive questions or questions that may drain their energy and mind
v. New or revised financial conflict of interest. Example:
-
- Change of sponsoring institution or legal representative of sponsor

